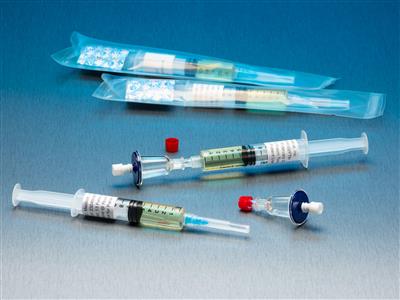
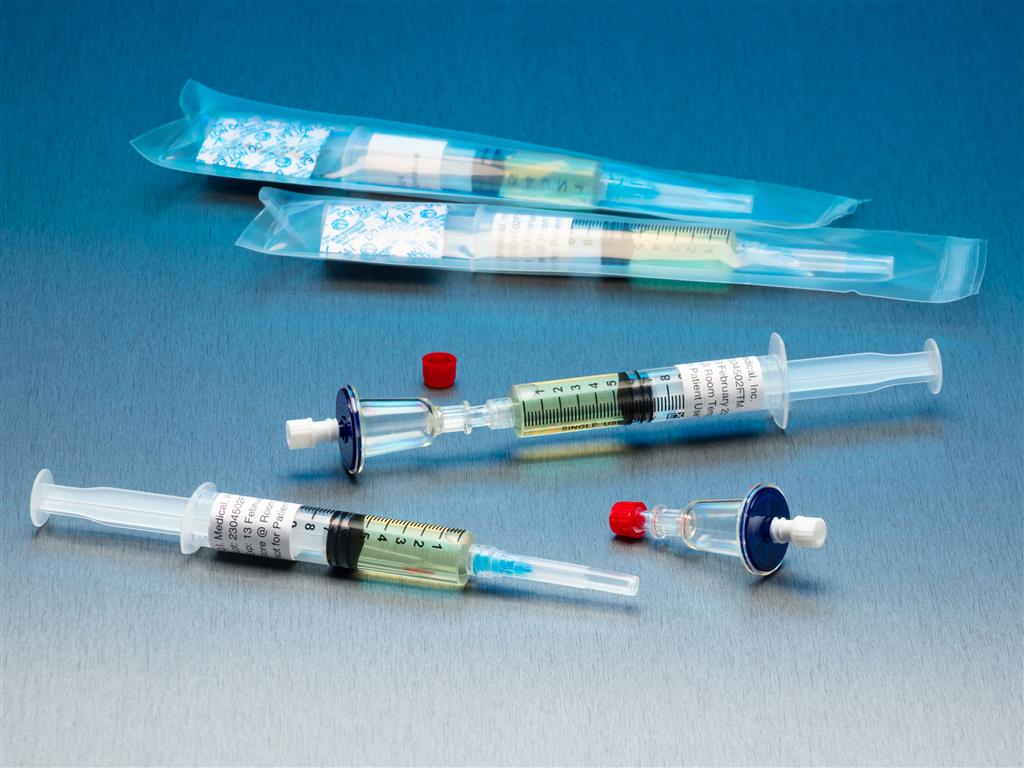
QT Micro 0.2m Contamination Tester, 5mL FTM Syringe, Female LL Inlet, Male Luer Slip Outlet
Item No.
TM6101
QT Micro 0.2m Contamination Tester Full microbial contamination test kit for small volume compounded sterile products. Nondestructive microbial testing kit allows for sample to be used after testing. FDA Registered Class 2 Device with required FDA Unique Device Identifier (UDI) barcode. 0.22µ
Description
QT Micro 0.2m Contamination Tester
Full microbial contamination test kit for small volume compounded sterile products. Nondestructive microbial testing kit allows for sample to be used after testing. FDA Registered Class 2 Device with required FDA Unique Device Identifier (UDI) barcode.- 0.22µ contamination tester for syringe to sterile transfers.
- 10 x QT Micro filter units with female luer lock inlet and male luer slip outlet
- 10 x 5cc syringes of FTM growth media
- Log sheet included
- Gummed labels included
- 10 units per case
Attachments
Reviews (0)
There are no reviews yet.